top of page
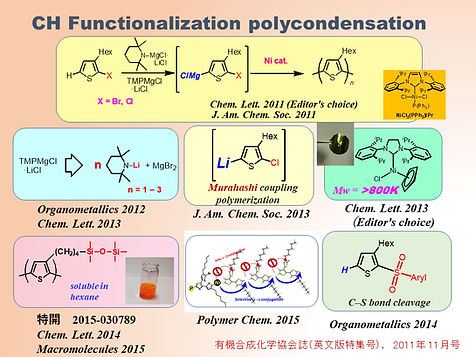
Polythiophene Synthesis
Employment of bulky magnesium amide such as Knochel-Hauser base to the reaction of 2-halo-3-substituted thiophene undergoes deprotonation at the 5-position. Following addition of a nickel(II) catalyst induces polymerization to afford head-to-tail-type highly regioregular polythiophenes in a facile manner. 1. dehydrobrominative polymerization 2. Chlorothiophene is also available!! Combination of RMgX and catalytic R2NH can also be employed instead. 3. Synthesis of poly(thienylene arylene)s 4. Extremely high-molecular-weight polythiophene (Mw: higher then 800 KDa; Photo: Self-standing film of thus obtained polythiophene) 5. Combination of lithium amide and MgX2 (ratio:1-3) wil also be replaced with Knochel-Hauser base 6. Formation of thiophene-Li species: Murahashi coupling polymerization. 7. Cross-coupling polymerization via C-S bond cleavage.
Review:
J. Synth. Org. Chem. Jpn., 2011, 69 (11: special issue in English),
1202-1211 OPEN ACCESS
Mini review:
Chem. Eur. J. 2020, in press (10.1002/chem.201905653)
Recent papers:
Angew. Chem. Int. Ed. 2019, 58, 9547
Macromolecules, 2020, 53, 1171
bottom of page
